Transcribed Image Text
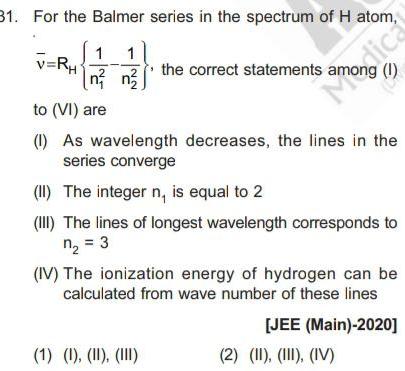
31 For the Balmer series in the spectrum of H atom 1 1 n n v RH the correct statements amodica 1 M to VI are 1 As wavelength decreases the lines in the series converge II The integer n is equal to 2 III The lines of longest wavelength corresponds to n 3 1 1 II III IV The ionization energy of hydrogen can be calculated from wave number of these lines JEE Main 2020 2 II III IV
Other questions asked by students
Biology
Calculus
Accounting
Accounting
Accounting
Accounting